雀巢(Nestle)
Nestle Oral Impact™ Coffee Flavour (237ml*3 packs)
Nestle Oral Impact™ Coffee Flavour (237ml*3 packs)
Couldn't load pickup availability
Nestle Oral Impact™ Coffee Flavour (237ml*3 packs)
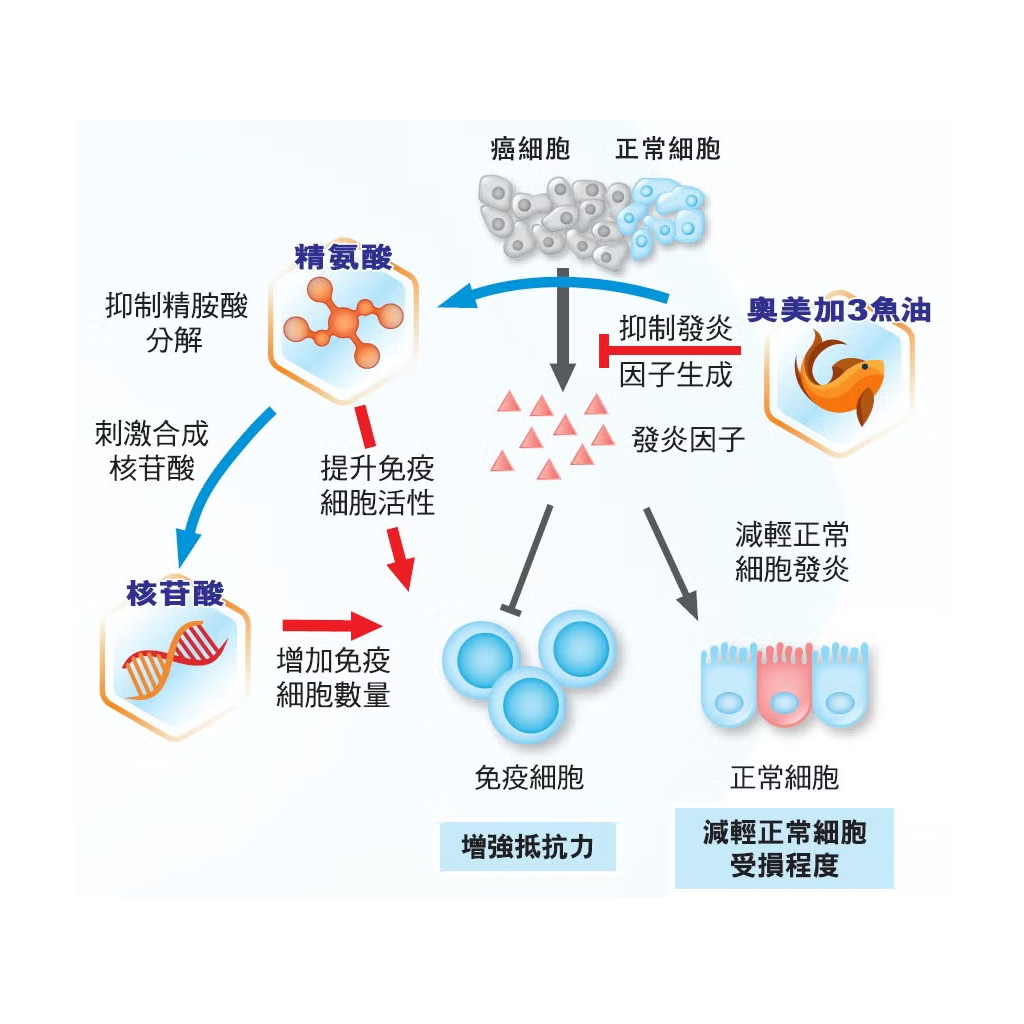
Oral Impact™ is a balanced and complete nutritional supplement for cancer treatment. Its formula specifically contains Arginine, Nucleotides and Omega-3 fish oil. Clinical studies have proven that it can improve the resistance of cancer patients1, help patients complete cancer treatment on time and alleviate the side effects of chemotherapy and radiotherapy. After completing all cancer treatments, patients also need to continue to use Oral Impact™nutritional supplements to maintain good immunity.
Oral Impact™ is delicious and can be used as a meal replacement for breakfast, afternoon tea, snacks, after-dinner desserts and supper. The newly launched ready-to-drink package is convenient for drinking. In addition to the original tropical fruit flavor, it has been updated with a new coffee flavor to take care of cancer patients who like to drink coffee.
- Unique immune nutrition – contains arginine, nucleotides and omega-3 fish oil
- Clinical evidence can improve the immunity of cancer patients1
- Significantly increases white blood cells and T lymphocytes in cancer patients2
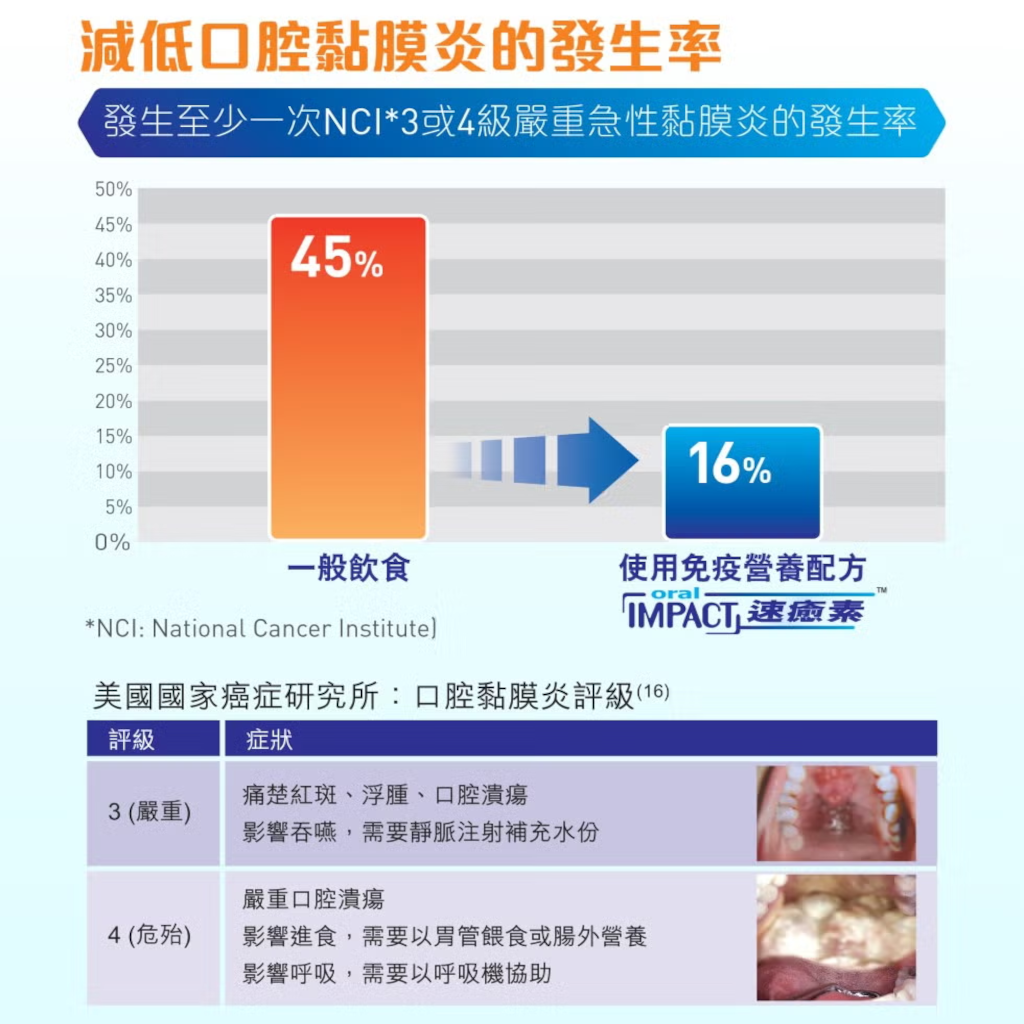
- Helps reduce oral mucositis3
- Helps patients complete cancer treatment on time and alleviate side effects during chemotherapy and radiotherapy
- Each pack provides 303 kcal of energy; cancer patients need to consume enough energy to maintain their weight
- Tropical fruit and coffee flavors, delicious
- Made in Germany
Suitable for people
- Cancer patients receiving electrotherapy, chemotherapy, surgery, and targeted drug treatment
- Patients undergoing major surgery
- Trauma patients
- Malnourished cancer patients
- Special medical food. Consume as directed by your doctor or healthcare provider.
Recommended serving size:
- 3 sachets per day for 5 days before treatment*
- 2 sachets per day during treatment*
- Powder preparation method: 250ml warm or chilled water + one pack (74g) of powder
* Treatment : electrotherapy, chemotherapy and surgery
Ingredients :
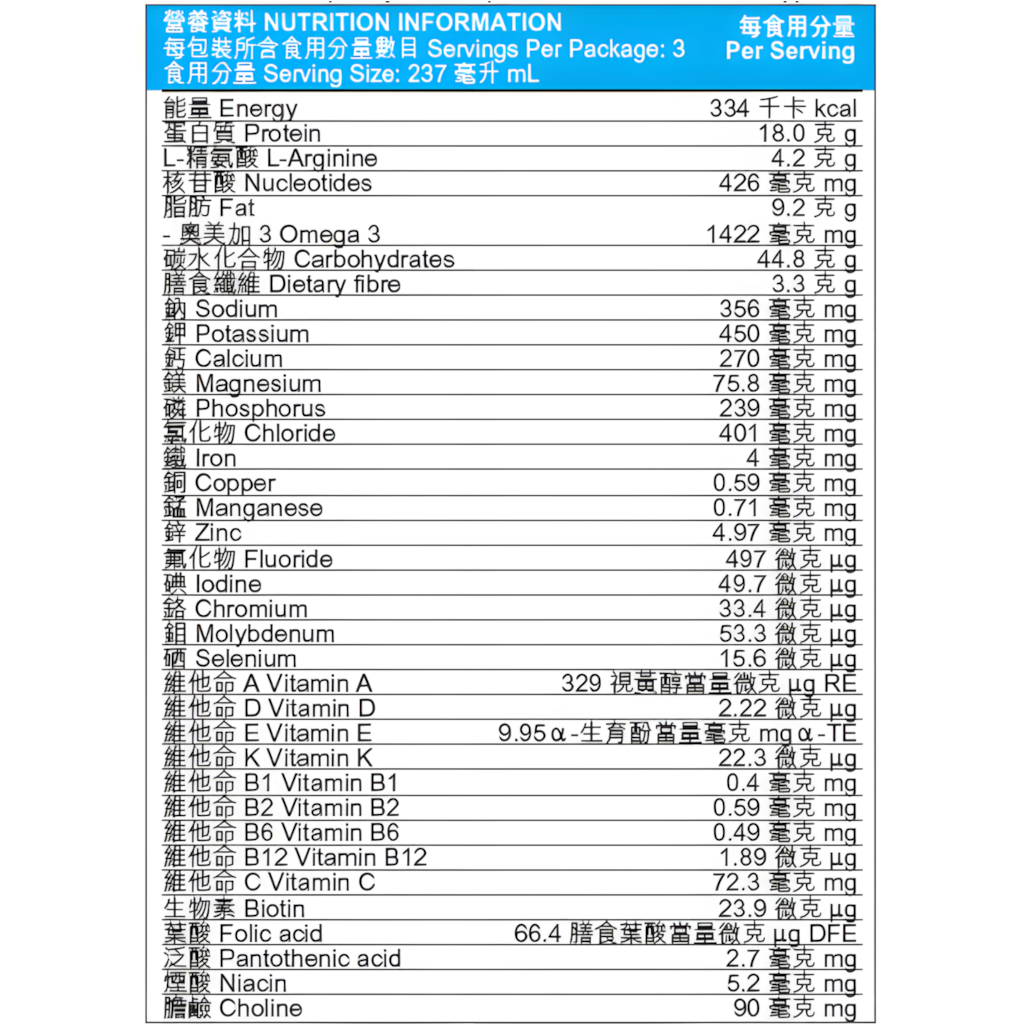
Water, sugar, maltodextrin, sodium caseinate, calcium caseinate, L-arginine (1.69%), fiber (guar gum), fish oil (1.5%), minerals (potassium citrate, citric acid sodium, calcium phosphate, sodium chloride, calcium citrate, magnesium chloride, potassium chloride, magnesium phosphate, iron pyrophosphate, zinc sulfate, copper gluconate, manganese sulfate, sodium fluoride, chromium chloride, sodium molybdate, potassium iodide, sodium selenate), medium chain triglycerides (1%), corn oil, acidity regulator (E330), decaffeinated instant coffee, emulsifier (E471, E322), sodium ribonucleotide (0.19%) , flavoring, choline bitartrate, vitamins (C, niacin, B5, E, B6, B2, B1, A, folic acid, biotin, K1, D3, B12), stabilizers (E460, E407, E466) , Antioxidant (E306), pigment (E160a).
Allergen warning: Contains milk, fish and soy products.
#According to Nielsen’s retail survey report on the cancer / chemotherapy / electrotherapy/ category-specific nutritional products in supermarkets, pharmacies and convenience stores in Hong Kong from August 2013 to July 2018 (©2018 Nielsen Company. All rights reserved)
+Oral Impact™ has been awarded the most popular health nutritional product (immune nutrition formula) among medical staff for 7 consecutiv years from 2012 to 2018 (organizer: Hong Kong Medical Association)
1. Daly MD, Weintraub FN, Shou J, et al. Internal Nutrition During Multimodality Therapy in Upper Gastrointestinal Cancer Patients. Annals of Surgery 1995; vol.221, No.4, 327-338
2. Maruyama T et al. Immunonutritional diet modulates natural killer cell activation and Th17 cell distribution in patients with gastric and esophageal cancer. Nutrition 2011. 27 146-152
3. Machon C, et al. Immunonutrition before and during radiochemotherapy: Improvement of inflammatory parameters in head and neck cancer patients. Support Care Cancer 2012;20:3129-3135
4. Rodriguez PC, et al. J Biol Chem. 2002;277(24):21123-9.
5. Munder M. Br J Pharmacol. 2009;158(3):638-51.
6. Grimble GK et al. Curr Opin in Clin Nutr Metab Care. 2001; 4(1):57-64.
7. Calder PC. Biochem Soc Trans 2005;33:423-427.5. Daly MD, Weintraub FN, Shou J, et al. Annals of Surgery 1995; Vol.221(4): 327-338.